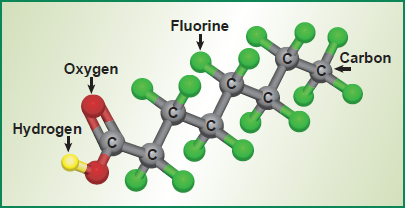
Perfluorinated chemicals (PFCs) and more specifically per- and polyfluoroalkyl substances (PFAS) are commonly found in cleaners, textiles, leather, paper and paints, fire-fighting foams, surfactants, and wire insulation. These persistent compounds have been found at trace levels to be in the environment and to bioaccumulate in wildlife and humans.
PFAS are generally carbon chains of varying length and can include varying amounts of oxygen, hydrogen, and nitrogen.
Whether a compound is considered a "per" or a "poly" depends upon the following:
- Per FAS - all the carbons in the change are bonded with fluorine
- Poly FAS - not all carbons in the chain are bonded with fluorine
PFAS have been in the news both locally and nationally as an emerging class of groundwater contaminants. Additional information associated with the extent of groundwater impacts and the chemical properties of these compounds can be found here and here. The most common of these compounds in the news contain 8 carbon atoms and are perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS). While there are potentially thousands of PFAS, some other common and emerging compounds include:
- Perfluorohexane sulfanate (PFHxS)
- Perfluorononanoic acid (PFNA)
- Perfluorobutanesulfonic acid (PFBS)
- GenX
Chemical properties generally common to PFAS are:
- Water soluble
- Low volatility
- Resistant to biodegradation
XDD Environmental has given numerous presentations and webinars associated with remedial options and unknowns for PFAS. A sampling of some of our presentations and webinars:
April 2018 Webinar on:
- PFAS Properties and Remedial Screening
- PFAS Remediation Details and Case Studies
2018 Amhest Soils Conference - Full Presentation
XDD Environmental's on-site treatability testing laboratory conducts original research into remedial options for PFAS. Currently XDD is partnering with GHD to evaluate several promising remedial approaches to PFAS using adsorption and ion exchange, including:
- Granular activated carbon
- Organically modified media
- Surfactant media
- Cationic blends
- Commercial blends
- Chemically pre-treated media
XDD played a critical role in project planning, execution and problem solving. They were instrumental to the success of the project.
Chemical/Physical Properties of PFOA and PFOS
| Property | PFOA | PFOS | Benzene |
| Chemical Formula | C8HF15O2 | C8HF17O3S | C6H6 |
| Molecular Weight(g/mol) | 414.09 | 500.13 | 78.11 |
| Boiling Point (oC) | 192.4 | 259 | 80 |
| Vapor Pressure (mm Hg at 25 oC) | 0.525 | ~0.002 | 86 |
| Henry’s Law Constant @ 25 oC (unitless) | Not measurable |
Not measurable |
0.225 |
| Koc | 115 | 371 | 79 |
| Solubility in Water (mg/L) | ~9,500 | 680 | 1,780 |
NM Not Measurable
PFOA Properties USEPA 2016
PFOS Properties USEPA 2016